Abstract
Introduction: Although the considerable advances have been made in the treatment of acute myeloid leukemia (AML) in recent decades, only one third of patients (except for acute promyelocytic leukemia) can be cured , and most suffer relapse or primary refractory (R/R). However, there is no standard regimen for R/R AML patients after conventional chemotherapy.A regimen consisting of granulocyte colony-stimulating factor (G-CSF) priming the combination of low-dose cytarabine (Ara-C) and aclarubicin, termed the CAG regimen,was first reported by a Japanese group in 1995. It has been proved that cladribine is a attractive drug in AML because of their significant synergy with other chemotherapeutic agents and favorable toxicity profile. Efforts were made to improve the efficacy of the CAG regimen among institutions, including combining it with other chemotherapy drugs. Therefore, the purpose of this study is to prospectively evaluate efficacy and safety of C-CAG regimen (cladribine in combination with granulocyte colony-stimulating factor, low-dose cytarabine and aclarubicin) in patients with R/R AML(This study was registered at www.chictr.org, the Clinical Trial Registration Number was ChiCTR-OPC-16010166).
Methods: Enrolment began in August 2016 for a Phase II single-center clinical trial . The patients in this arm will receive C-CAG regimen for salvage treatment,detailed as following:Cladribine 5mg/㎡,d1-5; G-CSF 300ug,d0-9; aclarubicin 10mg,d3-6;cytarabine 10mg/㎡ q12h, SC, d3-9;4 weeks a cycle.The patients are permitted to quit the study if complete remission(CR) is not achieved after 2 course of chemotherapy. If conditions were right,the patients achieving CR were recommended to receive allogeneic hematopoietic stem cell transplantation(HSCT). Otherwise, the patients were given for a total of six cycles unless there was disease progression or unacceptable side effects, or withdrawal of patient consent.
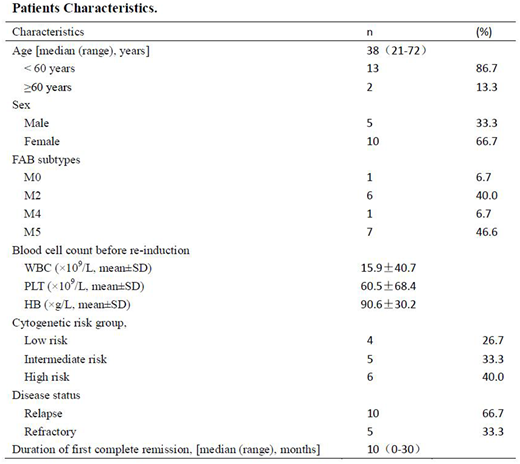
Results: Until June 1, 2018, we have completed the enrolment of 15 patients with R/R AML. The main clinical characteristics of the 15 patients are presented in Table 1. The median age was 38 years and ranged from 21 to 72 years. The majority of patients presented with FAB subtype of M2 or M5. All patients(n=15) have begun treatment and are evaluable for response. Of the 15 patients, complete remission rates is 73.3% to C-CAG regimen. No patients received allogeneic transplantation due to scarcity of bone marrow donors or inability of the patients to afford the expensive medical treatment.At a median follow-up of 6 months (2-24), the median DFS and OS for all 15 patients were 8 and 12 months, respectively.The most common adverse effect was myelosuppression. The incidence of grade 3 or 4 hematological toxicity was 80.0%.The respective median duration of neutropenia (neutrophils < 0.5×109/L) was 12 days. The median time of platelet recovery over 50 × 109/L was 23 days.All patients presented an incidence of 13.3% for Grade 3 or 4 infectious toxicity. No treatment-related deaths occurred. Non-hematological side effects were mild. Grade 3 or 4 gastrointestinal side effects were rarely observed, owing to the prophylactic antiemetic drug administration.
Conclusions: Preliminary data indicate that the C-CAG regimen chemotherapy is significantly effective against R/R AML with a high remission rate and a low hematological toxicity, thus serves as an alternative treatment for R/R AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal